The mammalian carotid body (CB) senses decreases in blood oxygen (hypoxia) to increase breathing within seconds. In hypoxia, neuroendocrine cells in the CB called glomus cells are activated to signal to afferent nerves that are a branch of the glossopharyngeal nerve (cranial nerve IX). Signals ascending through this nerve are ultimately transmitted to respiratory rhythm generators in the brainstem to regulate breathing.
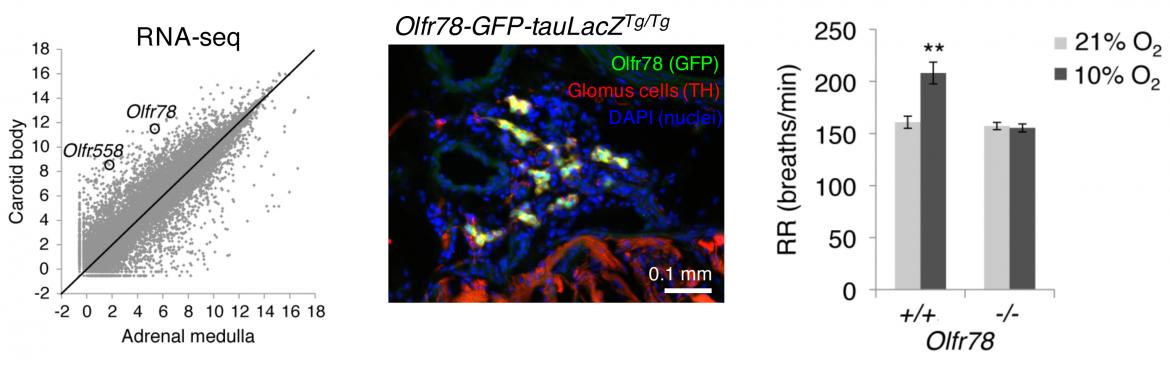
Even though the CB was first identified as an oxygen-sensing organ in the 1930's, the molecular mechanisms of oxygen sensing remains unclear. Taking a genomic approach using microarrays and RNA-sequencing, we identified an olfactory receptor (Olfr78) that is highly and selectively expressed in the mouse CB as a candidate oxygen sensor by comparing gene expression of the adult CB to the adult adrenal medulla, another neuroendocrine tissue with similar developmental origin as the CB but lacking acute oxygen sensitivity. Using mouse genetics, we confirmed expression of Olfr78 in CB glomus cells in Olfr78 reporter animals and found that Olfr78 null mutants have defects in ventilatory and CB sensory responses to hypoxia. This was a surprising result because olfactory receptors were traditionally thought to be expressed in the olfactory system for smell.
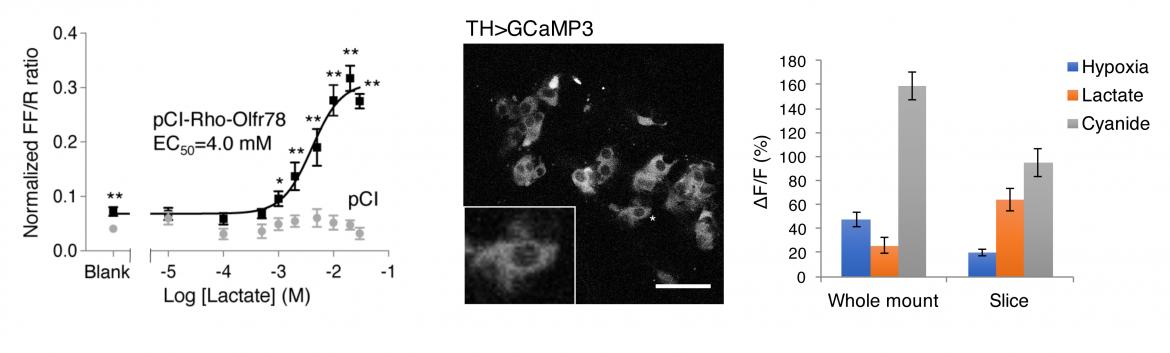
At a mechanistic level, we showed that Olfr78 receptor and CB glomus cells are activated by lactate, a metabolite generated acutely in hypoxia, by biochemistry and calcium imaging using a genetically encoded calcium indicator (GCaMP). We also demonstrated that lactate activation of the CB is dependent on Olfr78 by electrophysiology.
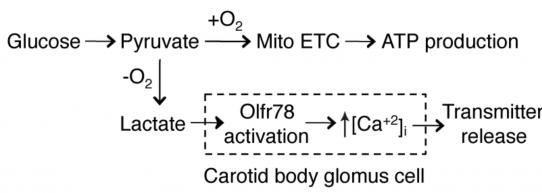
Our results support a model in which under normoxic conditions, pyruvate is efficiently used by the mitochondria for ATP production. However, in hypoxia pyruvate accumulation increases the production of lactate, which then activates Olfr78 on glomus cells to induce Ca+2 transients and neurotransmission to afferent nerves.
Currently, we are interested in the following questions:
1. What is the cellular and molecular mechanism of lactate production by the carotid body in acute hypoxia? Can we use newly developed molecular sensors to detect metabolic changes in the carotid body in real time?
2. Mitochondria of carotid body cells are exquisitely sensitive to changes in oxygen availability compared to those found in other cells. What underlies this unusual oxygen sensitivity?
3. How does lactate sensing through Olfr78 interact with other known pathways that contribute to acute oxygen sensing by the carotid body?
4. Can modulating molecular pathways for oxygen sensing in the carotid body improve symptoms of conditions associated with carotid body hyperactivity?